|
||||
REVISTA TRIPLOV
|
||||
|
||||
| DIREÇÃO | ||||
| Maria Estela Guedes | ||||
| Índice de Autores | ||||
| Série Anterior | ||||
| Nova Série | Página Principal | ||||
| SÍTIOS ALIADOS | ||||
| TriploII - Blog do TriploV | ||||
| TriploV | ||||
| Agulha Hispânica | ||||
| Arditura | ||||
| Bule, O | ||||
| Contrário do Tempo, O | ||||
| Domador de Sonhos | ||||
| Jornal de Poesia | ||||
|
ABSTRACT |
||||
|
Azolla is a small-leaf floating fern, which contains an endosymbiotic community living in the dorsal lobe cavity of the leaves. The presence in this cavity of a nitrogen-fixing filamentous cyanobacteria -Anabaena azollae - turns this symbiotic association into the only fern-cyanobacteria association that presents agricultural interest by the nitrogen input that this plant could introduce in the fields. In this work we review the applications and future challenges of the use of Azolla as biofertiliser in Africa. In this continent, agriculture is the most important sector of economy and it employs 75 % of the labour force. The dwelling of fossil fuel reserves and the increasing costs of commercial nitrogen fertilisers implicate finding other alternatives, such as the use of biofertilisers, like the Azolla-Anabaena symbiotic system. This plant is quite spread in the African continent. The taxonomy of Azolla is reviewed and the results of the cooperation project between Portugal and Guinea-Bissau for the use of this aquatic fern as green manure on rice cultivation are analysed. Finally, we focus the importance of the use of nitrogen-fixing organisms, like Azolla, which could help effectively developing countries to improve a more sustainable agriculture, without the risk of problems associated with the adverse effects of chemical fertilisers on long term soil fertility, soil productivity and environmental issues. |
||||
| RESUMO | ||||
|
Azolla é um pteridófito aquático que contém uma comunidade endossimbiótica vivendo na cavidade do lobo dorsal das folhas. A presença nesta cavidade duma cianobactéria filamentosa fixadora do azoto atmosférico -Anabaena azollae -confere a esta associação simbiótica grande interesse como biofertilizante em agricultura pela incorporação de azoto nos terrenos em que é utilizada. Neste trabalho são revistas as aplicações e futuros desafios do uso de Azolla como fertilizante natural em África. Neste continente, a agricultura é o sector mais importante da economia e emprega 75% da força laboral. O desgaste das reservas de combustíveis fósseis e o aumento do custo dos fertilizantes azotados de origem química implica encontrarmos alternativas, como o uso de biofertilizantes, nomeadamente o sistema simbiótico Azolla-Anabaena, o qual apresenta uma larga distribuição no continente Africano. A sistemática do pteridófito do género Azolla é revista e analisados os resultados do projecto de cooperação entre Portugal e a Guiné-Bissau para o uso desta planta como biofertilizante na cultura do arroz. Por fim, é realçado o papel dos organismos fixadores do azoto atmosférico, como é o caso de Azolla, no âmbito do desenvolvimento duma agricultura sustentável, sem o risco dos problemas associados aos efeitos adversos dos fertilizantes químicos na fertilidade e produtividade do solo a longo prazo, bem como em questões do foro ambiental. |
||||
| INTRODUCTION | ||||
|
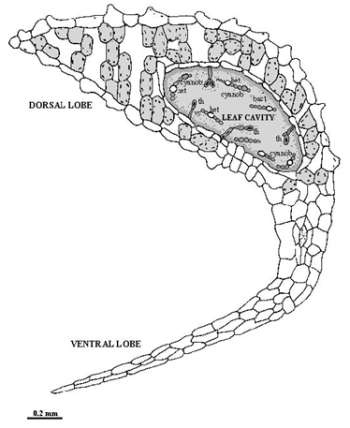
Agriculture is the most important sector of economy in Africa and it employs 75 % of the labour force of the continent. Two main agricultural systems domain this activity: a major traditional subsistence sector and a minor modern economical one. Sometimes those types can coexist in several countries. The traditional sector employs the majority of African’s rural population and is characterised by small and fragmented farms, little use of technology or fertiliser, high reliance on human labour, low yields, infrequent surpluses, and an emphasis on staple crops such as corn, rice, sweet potatoes, peanuts and other high-starch foods (Grolier Multimedia Encyclopedia, 1999). It is in this type of traditional sector that the problem of chemical fertilisers can be a restraining factor for agricultural development and crops’ production increase. The peasants subsistence type of agriculture prevents the existence of the necessary funds, namely to buy those chemicals and fuel and frequently contribute to the shortage of food (Dias and Carrapiço, 1991). In these conditions, which are associated with the dwelling of fossil fuel reserves and the increasing costs of commercial nitrogen fertilisers it is necessary to find others economical and technical options, that may contribute to solve or help this problem. One of these alternatives is the use of biofertilisers, especially associated with the use of plants symbiotic systems combined with the nitrogen fixation. It is the case of the aquatic fern of the genus Azolla, that presents a symbiotic association with a cyanobacterium -Anabaena azollae -and is quite spread in this continent. The aquatic fern of the genus Azolla is a small-leaf floating plant, which contains an endosymbiotic community living in the dorsal lobe cavity of the pteridophyte leaf (Figure 1). This community is composed of two type of prokaryotic organisms: one species of a nitrogen-fixing filamentous cyanobacteria -Anabaena azollae Strasb. (described by Strasburger in 1873) and a variety of bacteria that some identified as Arthrobacter sp. and associate with others showing the presence of nitrogenase (Costa et al., 1994). In this association, it is assumed that an exchange of metabolites, namely fixed nitrogen compounds, occurs from the cyanobiont to the host (Carrapiço and Tavares, 1989a, b). |
||||
|
|
||||
|
Fig. 1 – Transversal section of an Azolla leaf (Adapted from Sevillano et al., 1984). (th – transfer hair; cyanob – cyanobacterium; bact – bacteria; het – heterocyst; vc – vegetative cell. |
||||
|
Filaments of Anabaena azollae are localised in a cavity of the dorsal lobe of the fern's leaves, where special conditions stimulate high heterocyst frequency and a vegetative cell differentiation during leaf development (Carrapiço and Tavares, 1989a). The existence of the two symbionts inside the Azolla leaf cavity and its relationship with the fern, namely the metabolites flow between the host and the symbionts, can be seen as an unique micro-ecosystem with own well established caracteristics. This association is maintained during all the life cycle of the pteridophyte. The Anabaena apical colony is associated with shoot apex lacks heterocysts and, therefore, is unable to fix nitrogen. In mature leaves, the Anabaena filaments cease to grow and differentiate heterocysts, which are the site of N2 fixation. Besides the cyanobacteria, a population of bacteria undergoes a pattern of infection identical to Anabaena and probably is the third partner of this symbiosis (Wallace and Gates, 1986; Carrapiço and Tavares, 1989a; Carrapiço, 1991; Forni et al. 1989). The prokaryotic colony - cyanobacteria and bacteria - are also present in the sexual structures (sporocarps) of the fern (Carrapiço, 1991). The cyanobacterium is transferred from the sporophyte to the next generation via the megasporocarp. A cyanobacterium colony resides between the megasporocarp wall and the megasporagium one and inoculates the newly emerging sporophyte plant. A colony of the symbiotic cyanobacteria is formed near the shoot apex and thus enables symbiosis to be established within the developing leaf cavities (Watanabe and Van Hove, 1996). The presence of bacteria in the megasporocarps in association with the cyanobacteria also suggest a behaviour pattern similar to the cyanobionts (Carrapiço, 1991). The presence of Anabaena throughout the life cycle of the fern favours the obligatory nature of the symbiosis and suggest a parallel phylogenetic evolution of both partners (Watanabe and Van Hove, 1996). This symbiotic association is the only fern-cyanobacteria association that presents agricultural interest by the nitrogen input that this plant can introduce in the fields and continents (Moore, 1969; Kannayan, 1986; Van Hove and Diara, 1987; Shi and Hall, 1988; Wagner, 1997). Historically, Azolla has been used as green manure for wetland rice in northern Vietnam and central to southern China for centuries (Nierzwicki-Bauer, 1990; Watanabe and Van Hove, 1996). Only after the oil crisis in the 1970s the research and use of this type of association has been intensified because of the price increase of the chemical fertilisers and its negative impact in agriculture, namely in the countries of the third world (Dias and Carrapiço, 1991). Meanwhile, since the introduction of a market economy system in those countries, the increase on the supply of chemical fertilisers has reduced the traditional use of Azolla as green manure for rice cultivation, namely in China and Vietnam (Watanabe and Van Hove, 1996). A problem associated with the use of chemical fertilisers is the adverse effects on long term soil fertility, soil productivity and environmental safety (Kannaiyan, 1997). A new strategy for increasing rice production, particular in developing countries should be taken in account for programmes to utilise the biological fertilisers which will not only increase the rice productivity, but also improve the long term soil fertility (Kanniyan, 1997). In these conditions the inoculation of free living cyanobacteria in the fields is one of the options. The inoculation increased rice grain yields by an average of 350 kg/ha. When successful, the inoculation is low-cost technology, but its effect is erratic and unpredictable (Watanabe and Liu, 1992). More recently, a new method of using cyanobacteria on ammonia production for rice crop was developed by Kannaiyan’s team (Tamil Nadu Agricultural University, India), by means of immobilisation of nitrogen fixing Anabaena azollae and Anabaena variabilis in solid matrix of polyurethane and polyvinyl foam that excretes ammonia continuously with very positive results on rice culture ( Kannaiyan et al., 1996). For those reasons, Azolla use is yet a real option as a green fertiliser, especially in the developing countries that presents a low cost of labour force. In industrial countries, exploited for a more developing environmentally-friendly agricultural system (Watanabe and Van Hove, 1996), namely in particular segments of this important economical activity. Others uses of the Azolla-Anabaena system are now in progress, like the control of aquatic weeds, the use as animal feed and the concentration of mineral elements, namely its use as biofilter in industrial and domestical effluents (Watanabe and Van Hove, 1996; Costa et al, 1994, 1999; Lejeune et al., 1999; Tel-Or, personal communication). |
||||
|
Azolla TAXONOMY |
||||
|
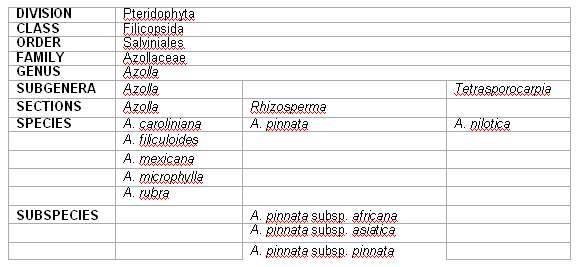
Despite its long history of agricultural use, Azolla taxonomy is controversial and our knowledge on the subject is still limited, which probably means that a new global revision of the taxon is required. In this paper we have used, what we think it is a coherent taxonomic classification (Saunders and Fowler, 1993), associated with the new research and progress data on the field, namely the studies using germplasm collections (Watanabe and Van Hove, 1996). The word Azolla has a Greek origin. It results from the agglutination of two words, azo and ollyo, which means killed by drought (Moore, 1969; Ashton and Walmsley, 1976). Indeed, it has a true predicative value indicating that these plants are very sensitive to water deficit. Azolla is a genus of aquatic ferns, mainly found in tropical and warm temperate regions. It can be observed in quiet waters, ponds, ditches, canals and paddy fields. Such areas may be seasonally covered by a mat of Azolla associated with other free-floating plants such as species of Lemna, Pistia, Salvinia, Trapa, Wolffia and mud rooting species of Cerathophyllum, Ludwigia, Neptunia and Polygonum. In Azolla the free-floating aquatic habit is exhibited by a complete adaptation of the whole sporophyte, including its reproductive structures. There is an extreme small plants present a branched stem that floats horizontally on the water surface, with single or fasciculate pendulous roots and alternately imbricate millimetric 2-lobed leaves. They are monoecious plants that possess dimorphic sporocarps, whose micro and megaspores develop in a leptosporangiate way. These ferns present a great disparity in their holomorphologies, being the most phenotipically influenced. This is another reason for the problems of Azolla systematics which are very ancient and evident in the different classifications proposed through the years. The genus has been established in 1783 by Lamarck and has been placed in the Salviniaceae along with the genus Salvinia (Svenson, 1944). Since then, it has been placed in the Marsileaceae, by R. Brown, in 1810 (Svenson, 1944) and again in the Salviniaceae by eminent systemats such as Sadebeck, in 1902, Lawrence, in 1951 and Benson, in 1957 (Ashton and Walmsley, 1984). The first to consider Azolla in the monotypic family Azollaceae was Wettstein, in 1903 (Bonnet, 1957) but only with Reed, in 1954, such proposal has begun to be accepted and followed (Ashton and Walmsley, 1984). The living species of Azolla are divided into two subgenera: Azolla and Rhizosperma (Mey.) Strasb. (Ashton and Walmsley, 1984). Some other authors use the taxonomic level section instead of subgenera (Moore, 1969; Saunders and Fowler, 1992). In both cases this separation is based in the floats number and in the massulae trichomes. All species with three floats in the megasporocarp and arrow-shaped glochidia belong to the subgenus Azolla. The specific classification, usually accepted, recognises five species integrated in the subgenus Azolla which are: A. caroliniana Willd., A. filiculoides Lam., A. mexicana Presl., A. microphylla Kaulf and A. rubra R. Br. All these taxa have a notorious morphologic resemblance. Subgenus Rhizosperma includes the species with nine floats in the megasporocarp and absent glochidia or with internal massulae trichomes. It comprises two species: A. subsp. africana (Desv.) R. M. K. Saunders and K. Fowler, A. pinnata subsp. asiatica (Desv.) R. M. K. Saunders & K. Fowler and A. pinnata subsp. pinnata. Some authors considered a third species in this subgenus, A. imbricata Nak. (Nakai, 1925), but it might be a synonymous of A. pinnata (Moore, 1969; Ashton and Walmsley, 1984) and, for others, it might be a synonymous of A. pinnata subsp. asiatica (Saunders and Fowler, 1992). In 1993, using cladistic, Saunders and Fowler proposed another supraspecific classification. They consider that the differences that divide A. nilotica from all the other Azolla species are enough to establish a new subgenus. That subgenus, denominated Tetrasporocarpia (sporocarps grouped in four), includes only A. nilotica. Subgenus Azolla, is then divided into two sections, Azolla and Rhizosperma, the last one conglobating just A. pinnata, this with 3 subspecies. Table 1 presents the last classification proposed by Saunders and Fowler (1993) and followed by Diniz and Carrapiço (1999). |
||||
|
|
||||
|
Table 1 -Synopsis of the classification of Azolla, proposed by Saunders and Fowler, 1993. |
||||
|
Azolla LIFE CYCLE |
||||
|
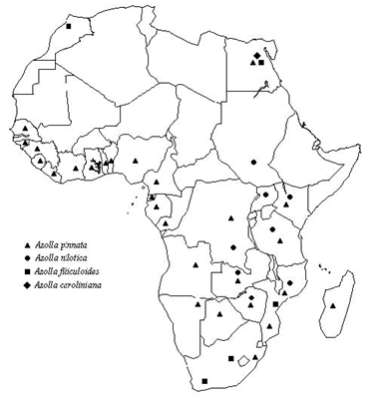
Azolla usually reproduces vegetatively by fragmentation of the abscision layer, at the base of each branch. Sexual reproduction is not very common and seems to be influenced by environmental factors, namely several stress conditions. Sporocarps are visible in pairs (in A. nilotica in four) in the place of the first leaf lower lobe, of a sporophyte branch. They occur in pairs of either microsporocarps, megasporocarps or one of each. Mature microsporocarps are globular and enclose numerous stalked microsporangia, each one with 32 or 64 microspores, divided into 3 to 10 complex structures, called massulae. In subgenus Azolla each massulae shows characteristic arrowlike projections, the glochidia. In subgenus Rhizosperma glochidia do not exist or there are filiform internal trichomes. At maturation microsporangia releases massulae which may be dragged underwater. Microspores germinate inside the massulae and flagellated antherozoids move through the gelatinised massulae to fertilise oospores within the archegonia (Braun-Howland and Nierzwicki-Bauer, 1990). Megasporocarps are much smaller than microsporocarps and have two distinct parts. The upper is filled by the so called floats, 3 in subgenus Azolla and 9 in subgenus Rhizosperma. In its lower portion, each megasporocarp contains only one megaspore. This germinates under water and a prothallus is formed producing chegonia. Antherozoids will fertilise the only egg cell existing in each archegonium and the zygote formation occurs within the megaspore apparatus below the water surface and will give rise to a new sporophyte (Braun-Howland and Nierzwicki-Bauer, 1990). Azolla is worldwide distributed and man influence is the main responsible for the dispersion of these plants through the globe. During the last two decades, the use of Azolla in rice cultivation has resulted in the introduction of new species to areas where they are not indigenous. New World species are now widespread in Asia and Africa, and often they had eliminated the natural populations of Azolla pinnata (Watanabe and Van Hove, 1996). Azolla filiculoides was introduced in the late 1970s into northern China where it is now a well adapted species (Watanabe and Van Hove, 1996). Before that action some species were endemic in the following areas: A. caroliniana, in eastern United States (Svenson, 1944); A. filiculoides, from Alaska to the southern South America (Svenson, 1944); A. mexicana, from west coast of the United States, into Mexico and Central America; A. microphylla, from tropical and sub-tropical zones of the American continent (Moore, 1969); A. nilotica, in Nile basin (Moore, 1969); A. pinnata, in tropical Africa, southern Africa and Madagascar (Moore, 1969). A new revision of Azolla world distribution, as previously reported by Moore (1969) and Lumpkin and Plucknett (1980), now needs a global revision (Watanabe and Van Hove, 1996). At the present state of our knowledge, A. caroliniana has been observed in Central and South America, in the east of the Andes and reached western Europe (Lumpkin, 1987) and has also been recently introduced in Egypt in the Nile Delta (Yanni et al., 1994). A. filiculoides is still in western America and it has been introduced in South Africa as well as in western Europe, China, Japan and southern Australia and New Zealand (Lumpkin and Plucknett, 1980; Lumpkin, 1987). A. mexicana has tolerated little expansion from his indigenous area and A. microphylla has been confined to the Galapos Islands (Lumpkin, 1987). A. nilotica is still an African species and can be found in eastern Africa, from Egypt to South Africa. A. pinnata, besides tropical Africa, is also distributed in Australasia and Southeast Asia. According to Saunders and Fowler (1992), geographical zones: A. pinnata subsp. africana, A. pinnata subsp. pinnata and A. pinnata subsp. asiatica. In an Africa map (Figure 2) we present, in a more detailed way, the distribution of the Azolla species in that continent, including the results of some Portuguese prospecting expeditions, as well as some herbarium material kept in Centro de Botânica, Lisbon (LISC) and from references mentioned bellow. |
||||
|
||||
|
Figure 2 – Distribution of the Azolla species in Africa. |
||||
|
To achieve botanical descriptions of Azolla African species we consulted the following works: Schelpe (1970); Schelpe and Diniz (1979); Schelpe and Nicola (1986); Tardieu-Blot (1964 a, b); Franco (1971); Almeida (1986); Yanni et al. (1994). Some new data are also introduced from our own research. AZOLLA Lam. Azolla Lam., Encycl. Méth., Bot. 1: 343 (1783). Genus description as for the family. According to species distribution, the true African Azolla taxa are A. nilotica and A. pinnata subsp. africana. As mentioned before, A. filiculoides and A. caroliana has been introduced into this continent. Key to the African species: 1 - Elongate plants up to 5 cm long; megasporangia with 3 floats; massulae with external arrowlike glochidia at the apex ------------------- 3 -Deltoid plants or, if elongate, with more than 6 cm long; megasporangia with 9 floats; massulae with internal filiform trichomes and absent glochidia ---------------2 |
||||
| AZOLLACEAE | ||||
|
Free-floating aquatic plants with pinnate branched rhizomes and single or fasciculate roots, node inserted. Alternately imbricate 2-lobed leaves, with an aerial chlorophylous upper lobe and a no chlorophylous submerged lower lobe. Sporocarps with thin walls, developed at the branch first leaf; microsporocarp with numerous microsporangia each one with its own pseudo-cellular structure, called massulae, where microspores germinate; megasporocarp with a single megasporangium involving a single megaspore with 3 or 9 floats in distal apex, similar to massulae. Female gametophyte submerged. It is a single-genus family. 2 - Deltoid plants up to 2 cm long; single roots or up to 3 roots per node; sporocarps in 2; internal filiform trichomes --------------------------- 1. A. pinnata -Elongate plants with more than 6 cm; fasciculate roots; sporocarps in 4; internal filiform trichomes and absent glochidia ---------------------------------2. A. nilotica 3- Massulae with external arrowlike glochidia at the apex, rarely septate ---------------------------------------------------------------------------------3. A. filiculoides -Massulae with external arrowlike glochidia at the apex, often septate --------------------------------------------------------------------------------- 4. A. caroliniana 1. Azolla pinnata R. Br. Prodr. Pl. Nov. Holl.: 167 (1810). Type from Australia. Subsp. africana (Desv.) R. M. K. Saunders and K. Fowler in Bot. J. Linn. Soc. 109: 351 (1992). Type from West Africa. (Figures 3 and 4). Azolla africana Desv. in Mém. Soc. Linn. Paris 6, 2: 178 (1827). Type from West Africa. Azolla guineensis Schumach. in Kongel. Dansk. Vid. Selsk. 4: 236 (1829). Type from West Africa. Azolla pinnata var. japonica (Franch and Sav.) French and Sav. in Enum. Pl. Jap. 2, 2: 612 (1878) pro parte. Type from Japan. Azolla pinnata var. africana (Desv.) Baker in J. Bot. 25: 101 (1886). Type from West Africa. Azolla japonica sensu Nak. in Bot. Mag. (Tokyo) 39: 184 (1925) non Franch. and Sav. pro parte. Deltoid plants with horizontal rhizome, minutely papillate, up to 2 cm long and 0.2 mm in diameter; single roots or up to 3, hairy, up to 3.5 cm long. Leaves 2-lobed with the upper lobe imbricate, up to 1.1 mm long, papillate, with a chlorophylous central portion, hyaline border with 3-4 cells layers, with acute apex; lower lobe similar in size in the stem ventral face in the place of the lower lobe of the first leave, covered by the upper lobe, spherical, about 1.7 mm in diameter, with numerous stalked microsporangia; microspores massulae with internal filiform trichomes; ovoid megasporocarp up to 0.8 mm long with a prominent dark brown apex with a single granular megaspore surmounted by 9 floats; granular perine surface; tuberculate excrescences. Distribution: Occurs in tropical Africa, from Gambia to Senegal, to east up to Kenya and Tanzania and southwards up to Natal, Transvaal and Botswana. Also in Madagascar. In ponds and quiet rivers. It has been introduced in Egypt (drainage canals of the Nile Delta) between 1977-80 for agricultural purposes |
||||
|
|
||||
|
Figure 3
– Azolla pinnata subsp. africana (Desv.) |
||||
|
|
||||
|
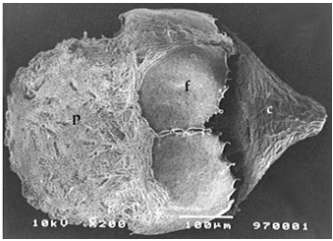
Figure 4 – Scanning electron micrograph of a megasporocarp of Azolla pinnata subsp. africana, with prominent apex and a granular megaspore perine surmounted by the floats (c-cap; f- float; p- perine). |
||||
|
2. A. nilotica Decne. ex Mett. in Kotschy and Peyr., Pl. Tinn.: 54, t. 25 (1867). Type from Central Africa. Elongate plants with horizontal rhizome or slightly ascending, pubescent, up to 35 cm long and 2 mm in diameter; numerous roots in 5 or more fascicles, hairy, up to 15 cm long. Leaves 2-lobed, usually with plane upper lobes and not imbricate, up to 2 mm long, broadly elliptic with a papillate chlorophylous central portion surrounded by broad hyaline margins, with acute or seldom obtuse apex; the lower lobe similar but smaller than the upper lobe. Microsporocarps and megasporocarps grouped in 4, either all megasporocarps, either all microsporocarps or mixed together, being initially enveloped by an hyaline ovoid membrane; microsporocarp up to 1 mm in diameter, spherical, with numerous stalked microsporangia; microspores massulae with internal filiform trichomes and eventually absent glochidia; megasporocarps up to 0.3 mm long, with a dark apex, with a single megaspore surmounted by 9 floats; slightly granular perine surface; sparse and small spiniform excrescences, specially near the distal pole. Sudan to Zimbabwe and Mozambique, up to the Zaire basin. In quiet waters and in lazy rivers. 3. Azolla filiculoides Lam., Encycl. Méth., Bot. 1: 343 (1783). Type from South America. This species differs from A. pinnata by presenting elongate plants, single node roots, leaf lobes with obtuse apex, unicellular papillae, more than two hyaline border cells, the glochidia have 0, 1 or 2 septa and the megaspore surmounted by 3 floats. The perine surface has raised hexagonal markings tied by the ends and microspore massulae with external arrowlike glochidia. Distribution: it occurs in western North, Central and South America. It has been introduced in South Africa (Free State and Cape Province) as well as in temperate western Europe and tropical and temperate China, Japan, Australia and New Zealand. In quiet waters, ponds and paddy fields. It has been introduced in Egypt (drainage canals of the Nile Delta) between 1977-80 for agricultural purposes. In South Africa, the uncontrolled growth of this fern is now a serious problem that in numerous water bodies, especially in the Free State, form a thick mat that put in risk the life in those ecosystems. 4. Azolla caroliniana Willd., Sp. Pl. 5 (1): 541 (1810). Type from North America. This species is similar to Azolla filiculoides from which it differs by presenting leaf lobes with acute apex, bicellular papillae, microspore massulae with external arrowlike usually septate glochidia, collar and megaspore perine surface densely covered with filosum. Distribution: it occurs in eastern North, Central and South America. It has been introduced in temperate western Europe and in Egypt (drainage canals of the Nile Delta) paddy fields. |
||||
| Azolla AS GREEN MANURE | ||||
|
The use of this fern as biofertiliser in Africa has been mainly implemented by ADRAO (Association pour le Développement de la Riziculture en Afrique de l’Ouest) and FAO (Food and Agriculture Organisation) in several Western African countries during the 1980s by a project co-ordinated by the Catholic University of Louvain, Belgium, with the support of the ADRAO field station located in St. Louis, Senegal (Diara et al., 1987; Van Hove and Diara, 1987; Van Hove, 1989). The goal of this project was to use the potential of Azolla as symbiotic nitrogen-fixing system for rice cultivation and to develop new agricultural techniques as well as testing several Azolla strains more adapted to local conditions. Despite some constraints, the results of this project were positive, not only for using Azolla as green manure, but also as feed for animals and namely through the development of an integrate use of Azolla with rice and animals farming, like fishes, pigs and ducks (Van Hove, 1989; Lejeune et al., 1999). Another region where Azolla was used is Egypt. From 1977 to 1980, soil microbiologists of the Agriculture Research Center, introduced three Azolla species: A. pinnata, A. caroliniana and A. filiculoides for green manuring of rice (Yanni et al., 1994). Two main procedures for Azolla application were used. Either the fern was grown on flooded field for 2-3 weeks and incorporated in the soil by ploughing often two weeks before rice plantation, or a dual culture method was used in which Azolla was added during the week after rice transplanting and the propagated Azolla incorporate in the soil after a temporary water drainage. The environmental and climatic conditions in Egypt indicates that the second method was the more adequate (Yanni et al., 1994). The results were better when Azolla was incorporated in soil rather when left floating during the all the rice growth season. With this technique the researchers could save half of the effects of nitrate and nitrite ions in water resources (Yanni et al., 1994). The use of molybdate to the inoculated rice field can enhance contribution of Azolla to rice performance. The Azolla species used in these field assays was A. caroliniana collected from water drainage canals. Two main problems appear to exist in the Azolla Egyptian project. The first one is the foregoing results indicating that the high labour cost needed for incorporation of Azolla is one of the most important constraints in all the project. The second one was and it is associated with the problem of Azolla escape during the field assays. This situation has occurred, namely during the field trials in season 1985, when Azolla escaped, undesignedly, to nearby drainage canals. Now, Azolla is present on most stagnant water of drainage canals in Nile Delta (Yanni et al., 1994). The uncontrolled growth of this fern is now a serious problem that in some places form a thick mat that put in risk the fish life present in those ecosystems and turn into a mechanical obstruction of irrigation and drainage of the canals. |
||||
|
Azolla IN GUINEA-BISSAU |
||||
|
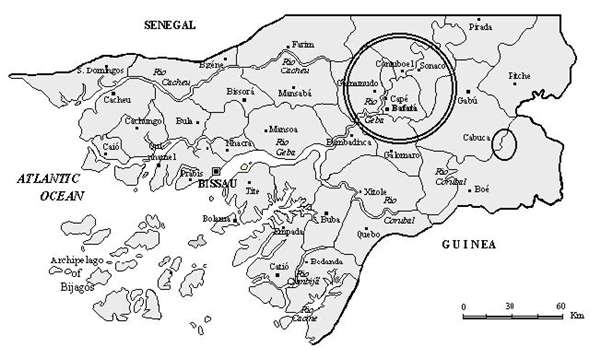
The Republic of Guinea-Bissau is a small West African country, located on the Atlantic coast between Senegal and Guinea (Figure 5), with an area of 36,125 Km2 and a population of 1 million inhabitants, with 850,000 persons working on agriculture. Most of the country is located on the African mainland, but also includes numerous offshore islands, most of them part of the Bijagós archipelago. The country is relatively flat, with low-lying areas subject to flooding. It includes a coastal plain, a somewhat higher interior plateau centered on Bafatá and a higher plateau near the border with Guinea (Boé region), where the land rises to 244 m. The coastal plain is made up of marshes and swamps and rain forests. Vegetation in the interior of the country is composed by is normally heavy from May to October and the dry season extends from November to April (Diniz and Carrapiço, 1999). The country’s coast is heavily indented by river estuaries and inlets. One of the more important rivers is the Geba that crosses the country from Northeast to West. This river has its spring in Senegal and its mouth in the Guinea-Bissau, together with the Corubal River, forming the Bissau estuary. In the eastern regions of these rivers, namely in the Geba, develops the aquatic fern Azolla pinnata subsp. africana., that forms, in the dry season, wide dark red carpets covering the river’s water (Diniz and Carrapiço, 1999) (Figure 6). This fern presents a vegetative cycle during several months of the year (August to March) and a sporulation period between November and March (Carrapiço (coord.) et al., 1996). |
||||
 |
||||
|
Fig. 5 - Distribution of Azolla pinnata in the Republic of Guinea-Bissau. |
||||
|
|
||||
|
Fig. 6 – Geba river
(Guinea-Bissau) almost covered |
||||
|
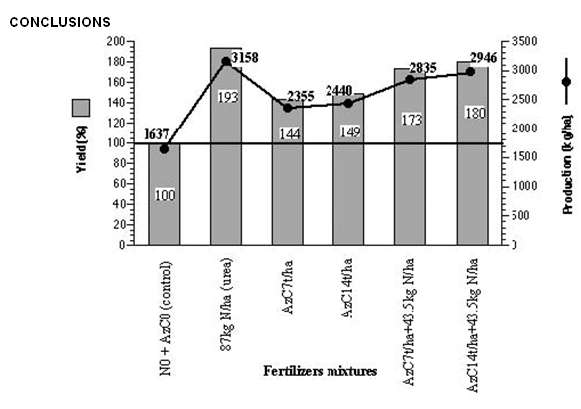
Guinea-Bissau is mainly an agrarian country and its economy is strongly influenced by the chemical fertilisers prices, which leads to a limitation of the farmer’s buying power, since most of them practices a livelihood agriculture. Rice culture is an important agrarian activity in the country and rice is one of the main feeding sources of the population (Dias and Carrapiço, 1991). This crop represents 65% of the country’s cereal production and their consumption per capita is estimated to be 167 kg/year. The difficulties they have to overcome to get these chemical fertilisers, that the country has to import from abroad, as well as the fragility of many of their soils, demands the study and the adoption of new solutions that, with the help of the local potentialities, would adapt to the crop’s techniques practised by the guinean farmers and would enable to keep the necessary crop productivity (Carrapiço (coord.) et al., 1996). During six years (1989-1995) a co-operation global project for the use of this aquatic fern as green manure on rice yield and improve the use of this biological system (Carrapiço (coord) et. al., 1996). The Azolla and rice experiments were developed in Contuboel (25 km from Bafatá) where the agronomic field station from National Institute for Agrarian Research (INPA) is located. Azolla pinnata was collected in the Geba river and maintained in the field station in small basins using superphosphate and furadan granules. The preparation of the field and the Azolla compost was done by the method suggested by Kannaiyan (1986) and also developed by ADRAO. The rice variety used during the years 1989, 1990, 1991 and 1995 was the BG 90-2 and the assays were done during the wet season (Figure 7). Different amounts of Azolla compost (7000 Kg/ha or 14000 Kg/ha), used 1 week before rice transplanting, were compared with urea fertiliser (87 kg N/ha). Some other experiments were conducted using a mixture of Azolla compost and urea -Azolla 7000 Kg/ha + 43.5 kg N/ha (urea) and Azolla 14000 Kg/ha + 43.5 kg N/ha (urea). All assays were compared with a blank treatment (no chemical nitrogen and no Azolla inoculation). The use of Azolla as compost was an option to avoid the problems associated with the uncontrolled growth of this fern and it was also chosen for its easy way to be used by the farmers. The experiences carried out on rice fertilisation (Figure 8) during those four years, show that the best results were obtained using only a chemical fertiliser, with an average production of 3158 Kg/ha of this crop. The second best production, 2946 Kg/ha, was obtained using a mixture of Azolla and urea, in the proportions of Azolla 14000/ha + 43.5 KgN/ha (urea) but a quite close result, 2835 Kg/ha was achieved using the mixture Azolla 7000 Kg/ha + 43.5 KgN/ha (urea). The production of the first mixture represents a yield of 180 % (expressed in function of the control assay), a value not far from the 193 % obtained with urea only. Considering the mixture Azolla 7000 Kg/ha + 43.5 KgN/ha (urea) the yield, 173% is quite similar to that mentioned above but represents a 50 % Azolla saving. |
||||
|
|
||||
|
Fig. 7
– A rice field assay at the Contuboel Center, |
||||
|
The global data was submitted to a one-way ANOVA analysis. It shows that the several fertilisers mixtures used were responsible for distinct rice yields (F= 6.09; p= 0.001) and the obtained results were consistent all along the experimental years (data was not a significant parameter F= 1.28; p= 0.176). In spite of the best results being obtained with urea, as it was expected, the prices of this chemical fertiliser and other productive factors strongly disagree with the exclusive use of it on rice fertilisation and suggest the use of Azolla compost combined with chemical fertiliser (Azolla 7000 Kg/ha + 43.5 kg N/ha) for increasing rice yield at low cost in this country. Considering the four years assays, we can say that the use of 7000Kg/ha Azolla compost has an effect equivalent to 43.5 Kg/ha of nitrogen, that is 94.5 Kg/ha of urea, with a production increase for the rice variety studied (BG-90-2) of 73 % compared with the control, in which the wet season is concerned. It means a 50% saving of the chemical fertiliser without significant loss on the rice yield production. |
||||
 |
||||
|
Fig. 8 – Average production (kg/ha) and yield (compared to control assay) of BG 90-2 rice variety relative to different fertilizers mixtures. Experimental data from GuineaBissau’s wet season during four years (1989, 1990, 1991 and 1995) was considered. |
||||
|
Despite the positive results obtained in the last recent years by nitrogen-fixing organisms in the field crop productivity, namely with the Azolla-Anabaena symbiosis, the gradual introduction of a more aggressive market economy in many African countries, the breakdown of the traditional agriculture way of living in some of them, namely with the population migration to the cities, associated with the pressure of the international industry, could lead some governments to increase the use of chemical fertilisers as the only solution for the problems of crop production and food shortage. We believe that it is help developing countries to improve a more sustainable agriculture, without the risk of problems associated with the adverse effects of chemical fertilisers on long term soil fertility, soil productivity and environmental issues. This strategy, including the creation of an Azolla research and development centre based in Africa suggested by Lejeune et al., (1999) can also help to fix the population and integrate it in new developing regional programmes that not only increase the crop productivity, but can also improve new applications of this fern in others economical sectors of activity. |
||||
| ACKNOWLEDGEMENTS | ||||
|
The authors are grateful to the Calouste Gulbenkian Foundation (FCG) and the National Board for Scientific and Technical Research (JNICT/FCT/MCT), Portugal, for its financial support in the Azolla Project with Guinea-Bissau and also to the President and the Azolla team of Guinea-Bissau’s National Institute for Agrarian Research (INPA). |
||||
| REFERENCES | ||||
|
ALMEIDA, M. T. (1986). Azolla Lam. In Flora Iberica. Real Jardín Botánico, C.S.I.C., Madrid. Vol I: 155-157. ASHTON, P. J. & WALMSLEY, R. D. (1976). The aquatic fern Azolla and its Anabaena symbiont. Endeavour. 35: 39-43. ASHTON, P. J. & WALMSLEY, R. D. (1984). The taxonomy and distribution of Azolla species in southern Africa. Botanical Journal of the Linnean Society. 89: 239-247. BONNET, A. L. M. (1957). Contribution à l’étude des hydropteridées. III - Recherches sur Azolla filiculoides. Revue de Cytologie et Biologie. 18: 1-85. BRAUN-HOWLAND, E. B. & NIERZWICKI-BAUER, S. A. (1990). Azolla-Anabaena symbiosis: biochemistry, physiology, ultrastructure, and molecular biology. In Florida: 65-117. CARRAPIÇO, F. & TAVARES, R. (1989a). New data on the Azolla - Anabaena symbiosis. I- Morphological and histochemical aspects. In Nitrogen Fixation with non-legumes. F.A. Skinner et al.(Eds.), Kluwer Academic Publishers: 89-94. CARRAPIÇO, F. & TAVARES, R. (1989b). New data on the Azolla - Anabaena symbiosis. II- Cytochemical and immunocytochemical aspects. In Nitrogen Fixation with non-legumes. F.A. Skinner et al.(Eds.), Kluwer Academic Publishers: 95-100. CARRAPIÇO, F. (1991). Are bacteria the third partner of the Azolla-Anabaena symbiosis? Plant and Soil. 137: 157-160. CARRAPIÇO, F. (coord.), TEIXEIRA, G. & DINIZ, M. A. (1996). Azolla. Projecto de Cooperação, FCUL / DBV, 1 CD-ROM. COSTA, M. L., CARRAPIÇO, F. & SANTOS, M. C. R. (1994). Biomass and growth characterization of Azolla filiculoides in natural and artificial environments. In Nitrogen Fixation with non-legumes. N.A. Hegazi, M. Fayez e M. Monib (Eds.), The American University in Cairo Press: 455 – 461. COSTA, M. L., SANTOS, M. C. R. & CARRAPIÇO, F.
(1999). Biomass characterization of Azolla filiculoides grown in natural
ecosystems and wastewater". Hydrobiologia, DIARA, H.F., Van BRANDT, H., DIOP, A. M. and Van Hove, C. (1987). Azolla and its use in rice culture in West Africa. In IRRI (Ed.), Azolla Utilization -Proceedings of Workshop on Azolla Use. Fujian, China, 1985: 147-152. DIAS, J. & CARRAPIÇO, F. (1991). Studies on rice production using Azolla as biofertiliser in Guinea-Bissau. In Nitrogen Fixation with non-legumes. M. Polsinelli, R. Materassi and M. Vincenzini (Eds.), Kluwer Academic Publishers: 541-542. Azolla pinnata in the Geba river (Guinea-Bissau). Garcia de Orta, Série Botânica. 14 (1): 89-92. FORNI, C., CAIOLA, G. & GENTILI, S. (1989). Bacteria in the Azolla-Anabaena symbiosis. In F. A. Skinner et al. (Eds.), Nitrogen Fixation with Non-Legumes, Kluwer Academic Publishers: 83-88. FRANCO, J. A. (1971). Nova Flora de Portugal, Vol I, 1ª ed., Lisboa. 658p. GROLIER MULTIMEDIA ENCYCLOPEDIA. (1999). Grolier Interactive Inc. 1 CD-ROM. KANNAIYAN, S. (1986). Studies on Azolla pinnata for rice crop. Research Journal of Plant Environment. 3: 1-16. KANNAIYAN, S., UMA, D., ARUNA, S. J. & KUMARI, S. M. P. (1996). Immobilization of nitrogen fixing cyanobacteria Anabaena azollae and Anabaena variabilis in solid matrix on ammonia production for rice crop. 7th International Symposium on N2 fixation with non-legumes. Faislabad, Pakistan (in press). KANNAIYAN, S. (1997). Summary report presented in the 2nd Meeting of EEC Project at Kings College, London. “On the use of immobilized cyanobacteria for continuous ammonia fertilization in rice production”. LEJEUNE, A., CAGAUAN, A. & VAN HOVE, C. (1999). Azolla research and development: Recent trends and priorities. Symbiosis, 27: 333-351. LUMPKIN, T. A. & PLUCKNETT, D. L. (1980). Azolla: Botany, physiology and use as a green manure. Economic Botany. 34: 111-153. LUMPKIN, T. A. (1987). Collection, maintenance and cultivation of Azolla. In Symbiotic Nitrogen Fixation Technology. Elkan G. H. (Eds.), Marcel Dekker, Inc., New York: 55-193. MOORE, A. W. (1969). Azolla: Biology and agronomic
significance. Botanical Review. NIERZWICKI-BAUER, S. A. (1990). Azolla-Anabaena symbiosis: Use in agriculture. In Handbook of Symbiotic Cyanobacteria. Amar N. Rai (Ed.). CRC Presse, Boca Raton, Florida: 119-136. SAUNDERS, R. M. K. & FOWLER, K. (1992). A morphological taxonomic revision of Azolla Lam. section Rhizosperma (Mey.) Mett. (Azollaceae). Botanical Journal of the Linnean Society. 109: 329-357. SAUNDERS, R. M. K. & FOWLER, K. (1993). The supraspecific taxonomy and evolution of the fern genus Azolla (Azollaceae). Plant Systematics & Evolution. 184: 175-193. SCHELPE, E. A. C. L. E. (1970). Pteridophyta. In A. W. Exell and E. Launert (Eds.), Flora Zambesiaca. London: 68-71. SCHELPE, E. A. C. L. E. & Diniz, M. A. (1979). Pteridophyta. Flora de Moçambique. Junta de Inv. Científicas do Ultramar. Lisboa: 67-70. SCHELPE, E. A. C. L. E. & Nicola, C. A. (1986). Pteridophyta. In O.H. Leistner (Ed.), Flora of Southern Africa. Pretoria: 66-68. SEVILLANO, F., SUBRAMANIAN, P. & RODRIGUEZ-BARRUECO, C. (1984). La association simbiotica fijadora de nitrogenio atmosferico Azolla-Anabaena. An CEBA Vol. II. SHI, D.-J. & Hall, D.O. (1988). The Azolla-Anabaena association: historical perspective, symbiosis and energy metabolism. The Botanical Review. 54: 353-386 SVENSON, H. K. (1944). The New World species of Azolla. American Fern Journal. 34: 69-84. TARDIEU-BLOT, M. L. (1964a). Flore du Cameroun. Muséum National d’Histoire Naturelle (Ed.), Paris. 3:55-56. TARDIEU-BLOT, M. L. (1964B). Flore du Gabon. Muséum National d’Histoire Naturelle (Ed.), Paris. 8:41-43. Van HOVE, C. & DIARA H. F. (1987). Azolla
introduction in African agriculture progress and problems. International
Rice Commission Newsletters. 36 : 1-4. WAGNER, G. M. (1997). Azolla: A review of its biology and utilization. The Botanical Review. 63: 1-26. WALLACE, W. H. & Gates, J. E. (1986). Identification of eubacteria isolated from leaf cavities of four species of the N-fixing Azolla fern as Arthrobacter Conn and Dimmick. Applied Environmental Microbiology. 52: 425-429. WATANABE, I. (1982). Azolla-Anabaena symbiosis - its physiology and use in tropical agriculture. In Microbiology of tropical soils and plant productivity. Y.R. Dommergues and H.G. Diem (Eds.). Martinus Nijhoff/Dr W. Junk Publishers, The Hague: 169-185. WATANABE, I. & Liu, C. C. (1992). Improving nitrogen-fixing systems and integrating them into sustainable rice farming. Plant and Soil.141: 57 - 62. WATANABE, I. & Van Hove, C. (1996). Phylogenetic, molecular and breeding aspects of Azolla-Anabaena symbiosis. In J. M. Camus, M. Gibby and R. J. Johns (eds.). Pteridology in Perspective. Royal Botanic Gardens, Kew: 611 – 619. YANNI, Y. G., SHALAAN, S. N. & El-Haddad, M. (1994). Potential role of Azolla as green manure for rice in Nile Delta under different levels of inorganic fertilization. In Nitrogen Fixation with non-legumes. N. A. Hegazi, M. Fayez and M. Monib (Eds.). The American University in Cairo Press: 127-132. |
||||
|
|
||||
|
(1) Francisco Carrapiço. Centro de Biologia Ambiental, Departamento de Biologia Vegetal, Faculdade de Ciências da Universidade de Lisboa, Edifício C2, Campo Grande, 1749-016 Lisboa, Portugal (E-mail: F.Carrapico@fc.ul.pt); (2) Generosa Teixeira Centro de Biologia Ambiental, Faculdade de Farmácia da Universidade de Lisboa, Avª das Forças Armadas, 1649-019 Lisboa, Portugal (E-mail:gteixeira@ff.ul.pt) & (3) M. Adélia Diniz. Centro de Botânica, Instituto de Investigação Científica Tropical, Trav. Conde da Ribeira 7-9, 1300-142 Lisboa, Portugal (E-mail: cbotn@iict.pt) |
||||
|
|
||||
|
© Maria Estela Guedes |
||||
|
|
||||